Authored by MD
Section 1: Introduction
Cosmetic gynecology is a rapidly growing field, fueled by technological advances and increasing societal interest in aesthetic and functional modifications of female genital anatomy. Among these innovations, Vagilangelo® is heavily marketed as a revolutionary, minimally invasive vaginal rejuvenation technique promising to restore the "natural vaginal angle" disrupted by childbirth or aging, with purported benefits in sexual satisfaction and sensitivity.
However, beneath its glossy promotional veneer lies a complex web of scientific uncertainties, ethical dilemmas, and health equity challenges. Today, we will dissect these layers with academic rigor and clinical insight to provide you, our listeners, with a nuanced understanding of Vagilangelo®’s place in modern gynecological practice.
Section 2: Critical Scientific Weaknesses and Evidence Gaps
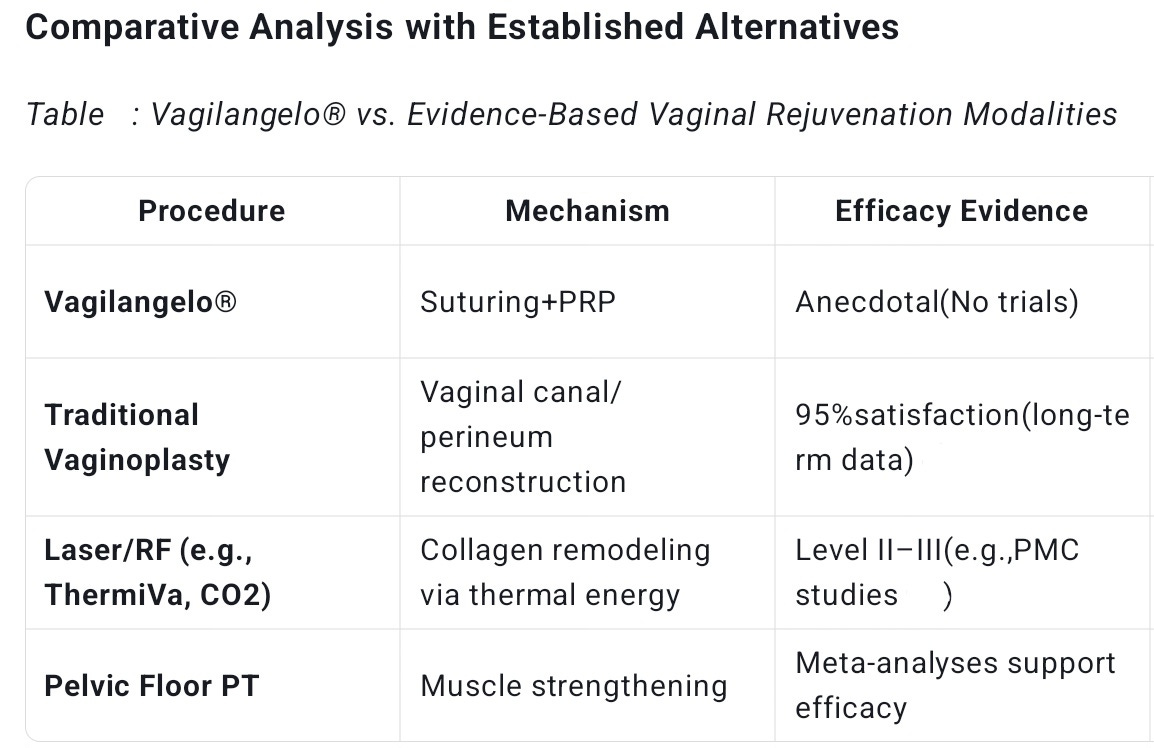
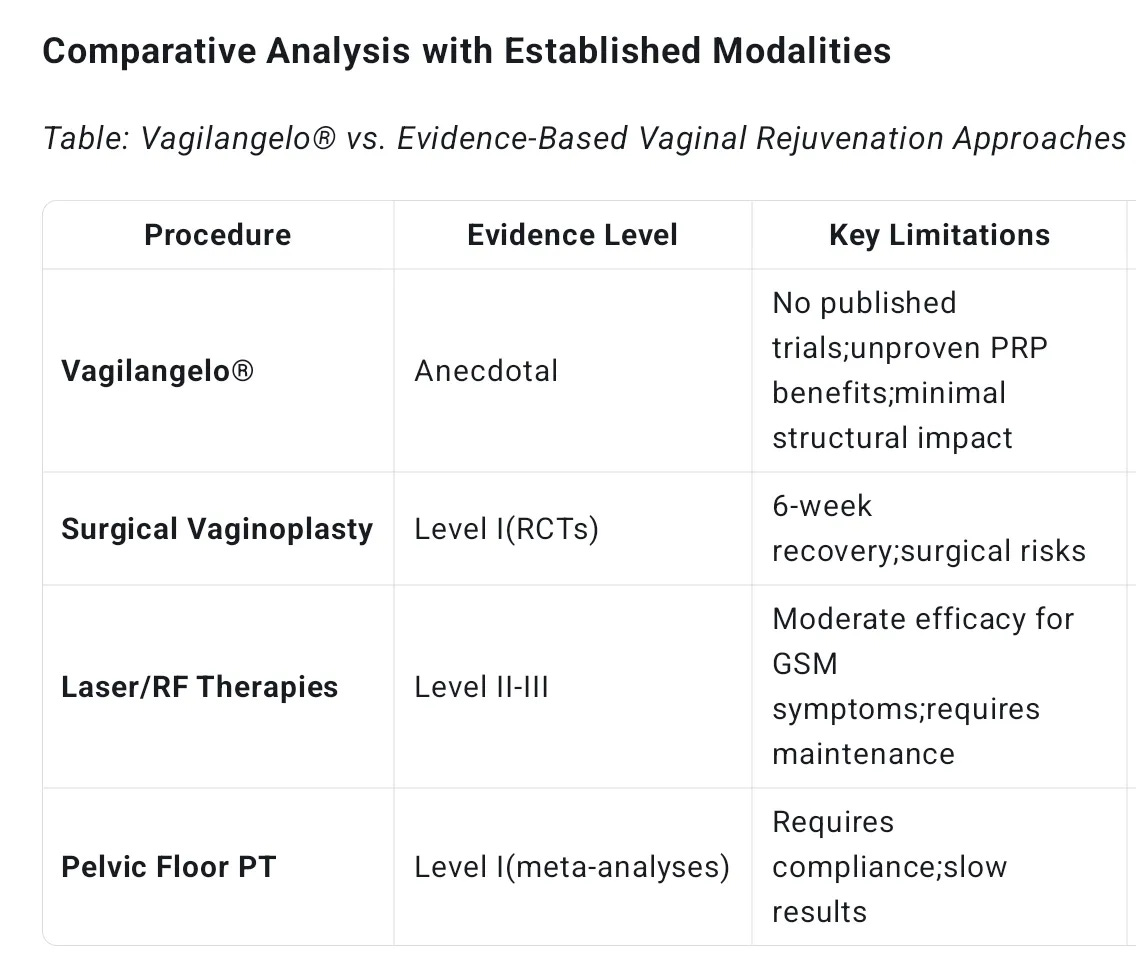
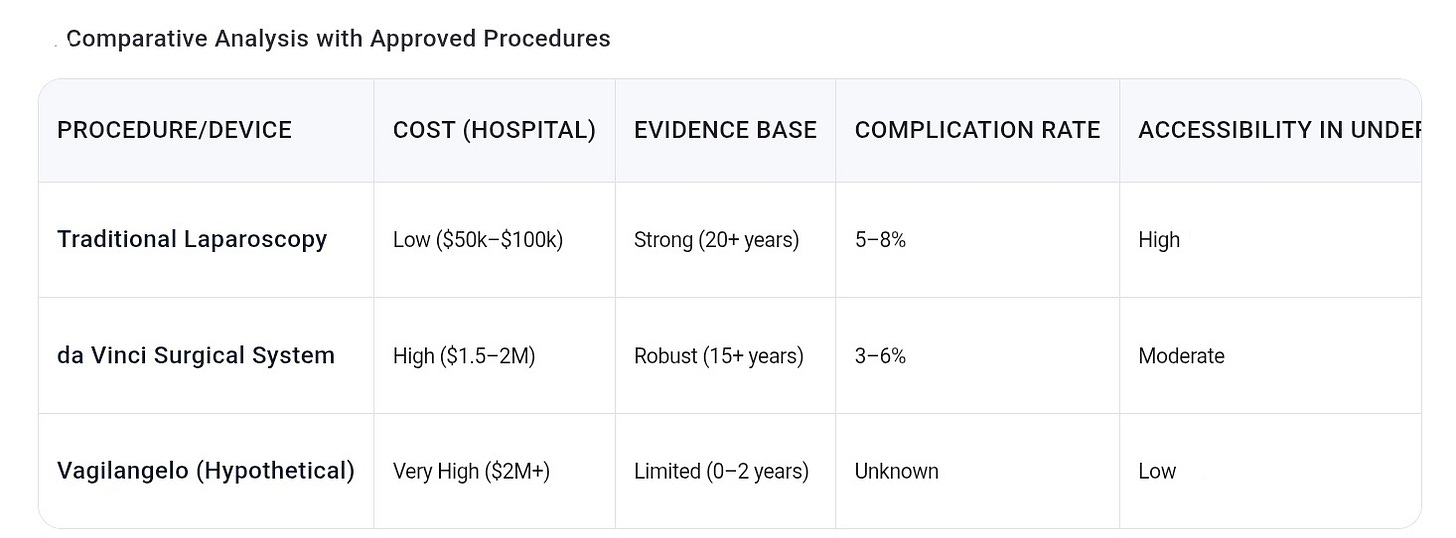
The most significant issue surrounding Vagilangelo® is the stark absence of rigorous clinical evidence. Unlike well-established vaginal rejuvenation procedures documented in peer-reviewed journals such as The Journal of Sexual Medicine or the American Journal of Obstetrics and Gynecology, Vagilangelo® relies almost exclusively on manufacturer websites and patient testimonials for its claims. There are no Level I to III studies—meaning no randomized controlled trials, cohort studies, or even case series—to definitively prove its efficacy or safety.
As Dr. Reza Lankarani, a respected clinical researcher, has emphasized, this lack of high-quality evidence is a glaring flaw that undermines the procedure’s credibility. In contrast, other less invasive modalities such as laser or radiofrequency (RF) therapies for vaginal rejuvenation have at least preliminary clinical data supporting their use.
Furthermore, the biological rationale for the use of PRP injections in Vagilangelo® is problematic. Platelet-Rich Plasma has demonstrated efficacy in some medical fields, such as orthopedics, but its role in vaginal tissue regeneration and sensitivity enhancement remains unproven. Scientific literature, including recent studies published in journals like Cells in 2023, highlights the variability in PRP composition, the lack of standardized injection protocols, and conflicting results regarding its benefits for vaginal lubrication or sensitivity.
Beyond these biological uncertainties, Vagilangelo® does not adequately address key structural issues. The procedure explicitly avoids correction of pelvic floor musculature or significant ligamentous laxity, which are often involved in pelvic organ prolapse or functional disorders. Marketing materials themselves acknowledge that Vagilangelo® offers less tightening than traditional vaginoplasty—rendering it unsuitable for patients with moderate to severe pelvic floor dysfunction.
Finally, the risks associated with Vagilangelo® are frequently downplayed. Although marketed as “non-invasive,” internal suturing near delicate pelvic nerves and organs carries inherent risks such as suture erosion, chronic pain (dyspareunia), and urinary symptoms (dysuria). PRP injections may also cause complications like infection, scarring, or paradoxical pain. Post-procedural care often requires sexual abstinence for several weeks, mirroring surgical aftercare, yet standardized protocols for managing complications are lacking.
---
Section 3: Comparative Limitations Against Established Alternatives
In the landscape of vaginal rejuvenation, Vagilangelo® occupies an ambiguous niche. It is less invasive than traditional surgical options like vaginoplasty but more invasive than energy-based modalities such as laser or radiofrequency therapies.
Energy-based devices have the advantage of inducing collagen remodeling with documented histological evidence and relatively low risk profiles, without the need for suturing. Vaginoplasty, on the other hand, is the gold standard for correcting severe anatomical defects, with decades of outcome and safety data.
Vagilangelo® lacks the evidence base, functional scope, and risk-mitigation protocols of these traditional methods. It is neither a substitute for surgery nor a clearly superior alternative to energy-based treatments. This “middle ground” status complicates clinical decision-making and patient counseling.
---
Section 4: Ethical Concerns and Commercial Exploitation
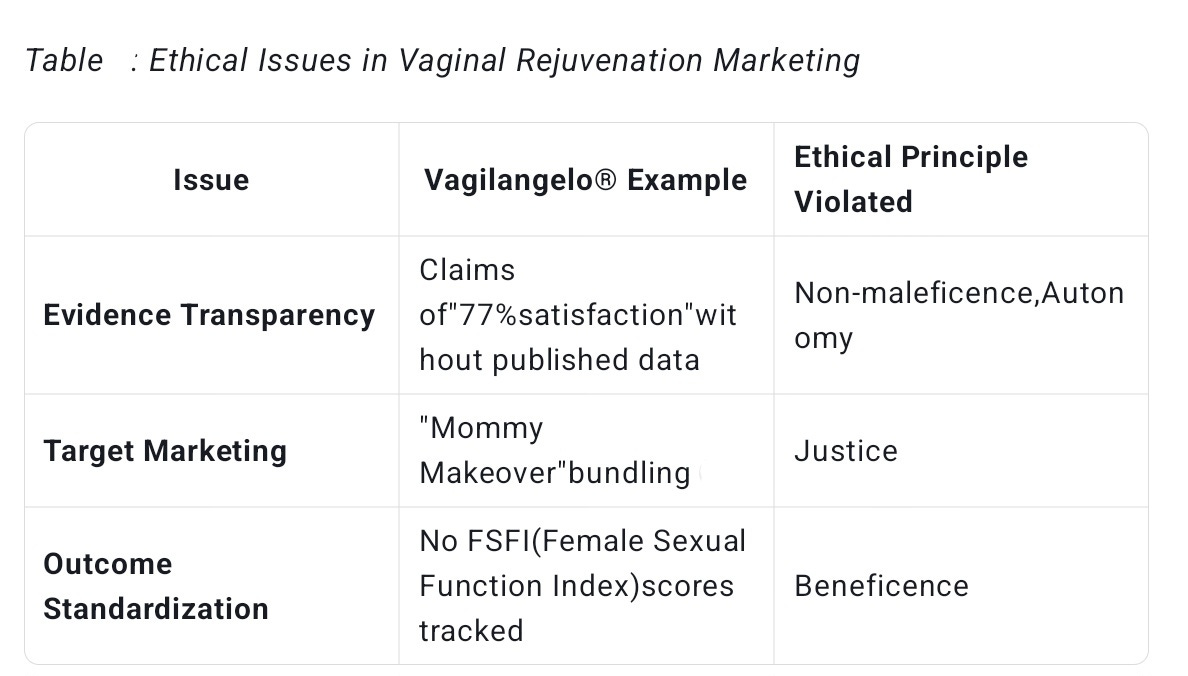
Turning to the ethical dimension, Vagilangelo® raises serious concerns about the commercialization of women’s health insecurities. The procedure is heavily marketed using terms like “revolutionary” and “groundbreaking,” which can create unrealistic expectations among vulnerable populations—particularly postpartum women who may be distressed by natural anatomical changes after childbirth.
This marketing strategy violates ethical standards such as the American Medical Association’s Code of Medical Ethics §8.063, which stresses the importance of providing patients with realistic information to facilitate informed consent. Without peer-reviewed data or transparent risk disclosures, patients cannot objectively weigh the benefits and harms, compromising their autonomy.
Moreover, the cost of Vagilangelo®—often exceeding $3,000 and typically paid out of pocket—limits access to affluent patients, exacerbating health disparities. Women from low-income backgrounds who might benefit more from functional pelvic floor repair remain excluded, highlighting a troubling pattern of “commercial health disparity” where profitable elective cosmetic procedures overshadow evidence-based care.
Another ethical issue is the medicalization of normal anatomical variation. Aging-related changes in vaginal structure are physiological, not pathological. Aggressively marketing angle correction risks pathologizing natural diversity in female anatomy, which can contribute to body dissatisfaction and unnecessary medical intervention.
---
Section 5: The Global Context – Cosmetic Tourism and Training Concerns
The Vagilangelo® controversy extends beyond individual patient care into systemic issues of global health inequity and medical commercialization. Bahrain serves as a case study illuminating the darker side of cosmetic gynecology tourism linked to Vagilangelo®.
Local tertiary centers in Bahrain report financial and clinical burdens from treating complications arising from cosmetic tourism procedures, including Vagilangelo®. These centers absorb substantial costs—estimated at $175,000 annually—managing infections, implant failures, and other adverse events. Meanwhile, patients often receive “all-inclusive” packages abroad that exclude meaningful postoperative care, leading to “patient abandonment” and additional strain on public health resources.
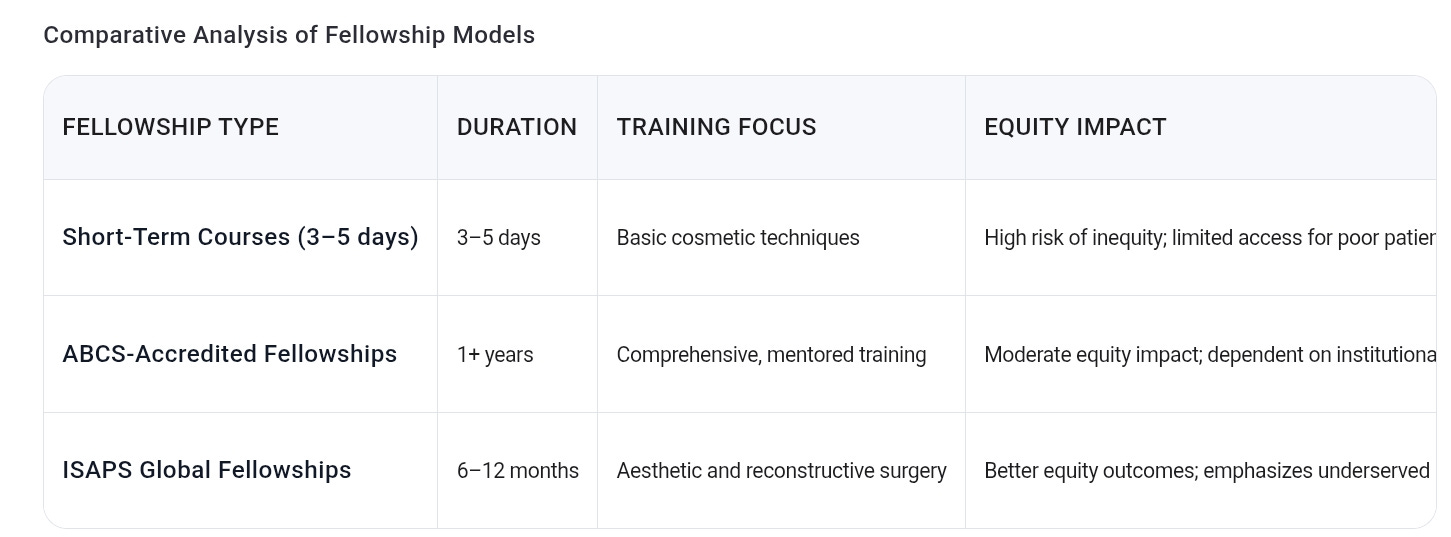
Furthermore, the training of surgeons performing Vagilangelo® and similar procedures in such regions is often brief and insufficient. Short-term cosmetic fellowships—sometimes lasting only weeks—fail to meet internationally accepted standards for supervised surgical skill acquisition. This contributes to ill-prepared practitioners performing complex interventions, increasing patient risk and eroding professional standards.
---
Section 6: Policy and Practice Recommendations
To address these multifaceted challenges, a series of rigorous policy and clinical recommendations are warranted:
1. Immediate Suspension of Direct-to-Consumer Advertising: No cosmetic gynecology procedure lacking robust randomized controlled trials and long-term safety data should be marketed directly to patients.
2. Conduct Comparative Effectiveness Research: Vagilangelo® must be evaluated in well-designed clinical trials against sham procedures, pelvic floor physical therapy, and established treatments to establish its true efficacy and safety.
3. Inclusion in Clinical Guidelines Only After Regulatory Approval: Professional societies and regulatory agencies should require FDA clearance and publication of peer-reviewed outcomes before endorsing Vagilangelo® in practice guidelines.
4. Prioritize Functional Restoration: Health systems and insurers should emphasize coverage for pelvic floor rehabilitation and functional surgeries over cosmetic interventions, promoting health equity.
5. Strengthen Surgical Training Standards: Cosmetic gynecologic fellowships must adhere to competency-based curricula, minimum duration requirements, and supervised clinical experience to assure patient safety.
6. Implement Ethical Commercialization Policies: Tax revenues from medical tourism profits should fund training scholarships and support public healthcare infrastructure in underserved regions.
7. Promote Global Partnerships: International academic collaborations can replace exploitative training models with sustainable “train-the-trainer” initiatives aligned with WHO recommendations.
---
Section 7: Conclusion – The Imperative of Evidence and Ethics
In summary, Vagilangelo® exemplifies the peril of innovation divorced from evidence and ethics. Its narrative reflects a broader trend in cosmetic gynecology—a pursuit of quick fixes and financial gain at the expense of scientific rigor and patient welfare.
As clinicians, researchers, and advocates for women’s health, our duty is clear: to champion interventions grounded in robust data, transparent risk communication, and equitable access. Until Vagilangelo® publishes outcomes commensurate with gold standard procedures, it must be regarded as experimental.
Our commitment is to anatomy, not angulation; to data, not dollars. Only through this lens can cosmetic gynecology evolve responsibly, respecting both the science and the dignity of the women.
M.D
















Share this post